Recently, Green Chemistry of the Royal Society of Chemistry published the paper "Construction and optimization of microbial cell factories for the sustainable production of bioactive dammarenediol-II glucosides" (Green Chem. 2019, 21(12): 3286-3299), which was achieved by Professor Ping Zhu's research group affiliated to the State Key Laboratory of Bioactive Substance and Function of Natural Medicines, Institute of Materia Medica, Chinese Academy of Medical Sciences and Peking Union Medical College. The co-first authors are Zong-Feng Hu & An-Di Gu, and the co-correspondent authors are Professors Jin-Ling Yang & Ping Zhu. The article described utilization of microbial cell factories for effectively producing the "unnatural" ginsenosides dammarenediol-II glucosides, which showed remarkable antitumor activity. The article was also selected as the back cover image of the present Issue (Fig. 1). Professor Yan Li at the Department of Pharmacology of our Institute carried out the evaluation test of the compounds.

Ginsenosides are the main bioactive components of Panax ginseng , a famous traditional Chinese medicine. The natural ginsenosides from this plant are dominantly present in the form of dammarane-type. These sapogenins are the glycosylation products of the two aglycones, protopanaxadiol (PPD) and protopanaxatriol (PPT), at the positions of C3-OH/C20-OH and C6-OH/C20-OH, respectively. Dammarenediol-II (DM) is the direct precursor of PPD, and PPD is further hydroxylated into PPT. Up to date, the DM glucosides have not yet been reported to be isolated from Panax species. Herein, researchers cloned the two UDP-glycosyltransferase genes, PgUGT74AE2 and UGTPg1 , from Panax ginseng , and expressed them by the E. coli system. Both the recombinant enzymes were found to be able to glycosylate DM into the "unnatural" DM glucosides 3β -O -Glc-DM and 20S -O -Glc-DM, respectively. In particular, the two ginsenosides showed remarkable in vitro and in vivo (mouse xenograft model) antitumor activity towards human colon carcinoma. To massively produce 3β -O -Glc-DM and 20S -O -Glc-DM by the de novo biosynthesis for the in-depth pharmacological evaluations, they refactored the complete biosynthetic pathways of 3β -O -Glc-DM and 20S -O -Glc-DM in Saccharomyces cerevisiae , respectively (Fig. 2A). The following strategies were applied to increase the production of the two products: optimizing the yeast chassis cell, up-regulating the corresponding enzymes in the biosynthetic pathway and inhibiting the branch pathway, multi-copy integrating the heterologous genes on the yeast genome through the CRISPR/Cas9 gene editing method, and over-expressing the transcription factor HAC1 (Figure 2B-D). Typically, the fed-batch fermentation was carried out in a 3-L fermenter by using the “exponential flow plus feeding” strategy, resulting in the titers of 2.4 g/L 3β -O -Glc-DM and 5.6 g/L 20S -O -Glc-DM (Fig. 2E, F), respectively.
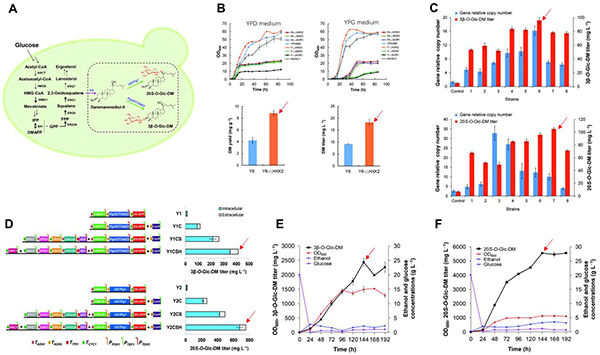
This study provides a new paradigm for utilizing synthetic biological methods in the production of natural products. It also generates a green and environmentally friendly approach for massively producing the aforementioned "unnatural" ginsenosides, and lays the foundation for the new drug research and development.
Full text:http://xlink.rsc.org/?DOI="C8GC04066D